Which of the Following Is a Weak Acid Chegg
Neither acidic nor basic. PKa is the negative logarithm to the base 10 of the dissociation constant of a weak acid.

Solved Which Of The Following Is A Weak Acid A Nitric Chegg Com
Which one of the following is a strong acid.

. Examples of weak acids are carbonic acid and ethanoic acid. HCN is less ionized hence less no of H View the full answer. Every weak acid has a unique pKa value.
The neutralization titration in this experiment is the reaction of an unknown weak acid HA with NaOH. Acetic acid C H 3 C O O H Formic acid H C O O H Carbonic acid H 2 C O 3 are considered as weak acids. View solution Give definitions.
Which of the following weak acids has the strongest conjugate base. HNO2 weak acid and NaNO3 Clear my choice To prepare a buffered solution with pH45 which pair of conjugate acid-base you should use. Select the best answer a.
-The molecular form of the weak acid does exist in solution. D HCl and HC2H3O2. HCO3 weak acid and NaCO3 b.
A HNO3 and H2CO3. Correct option is D Weak acids dissociates partially only eg in aqueous solution carbonic acid and acetic acid behave as weak acids. H3PO4 HNO2 H2SO3 HClO HClO2 HF H2S CH3COOH -Solutions of weak acids have a low concentration of H.
If HCl is added which ion will react with the extra hydrogen ions from the HCl to keep the pH from changing. A acetic acid Ka 18 10-5 B nitrous acid Ka 45 10-4 C dihydrogen phosphate ion Ka 62 10-8 D hydrocyanic acid Ka 40 10-10 E benzoic acid Ka 63 10-5. Click again to see term.
2 Which of the following statements is NOT true for weak bases. Which of the following statements is NOT true for strong acids. Acetone ethanol ethanal cyclohexanol phenol.
HA aq OH aq A aq H 2O 1 An acid-base titration can be monitored either through the use of an acid-base indicator or through the use of a pH meter. 1Among the given acids HCN is weak acid Hence option 3rd is correct option. Acetic acid or CH 3 COOH is a very common weak acid because it doesnt.
E HBr and H3PO4. For example the dissociation of a weak acid HA is written. Only small quantities of indicator are added to a solution.
CH3COOH weak acid and KCH3COO d. The correct answer is CH3COOH. The substance CH3CH22NH is considered to be A.
H2SO4 has a pKa1 of 3 so it dissociates very easily. The acid and base forms of the indicator are different colors. Often called acetic acid.
Acid-base indicators are derived from weak acids. Therefore pKa -log Ka. Tap card to see definition.
Click card to see definition. Of the following ______ is a weak acid HCl HBr HF HNO3 HClO4 This problem has been solved. C H2SO4 and H2S.
-Any acid that is not one of the seven strong is a weak acid eg. Which one of the following is a strong acid. The conjugate acid is BH and its strength is strong.
The ionization of carbonic acid in aqueous solution is shown below. View solution Explain the following. Which of the following compounds is a weak acid.
Hydrochloric acid in aqueous solution ionizes completely into hydrogen ions and chloride ions Weak acids are acids which ionizes only partially in aqueous solutions to hydrogen ions and the corresponding anions. HCI strong acid and NaCl c. Acids are classified as either strong or weak based on their ionization in water.
Of the following _____ is a weak acid HCl HBr HF HNO3 HClO4 Question. - 20309722 You found that the final temperature of the iced tea is between. Which of the following is a characteristic of a weak acid.
1 There are seven common strong acids. Which of the following statements concerning acid-base indicators are true. Each of the following pairs contains one strong acid and one weak acid except.
C H 3. A 1 only b 2 only c 3 only d 2 and 3 e 1 2 and 3. A weak acid is an acid which on dissociation in an aqueous solution produces low concentration of H ions.
A weak acid is an acid that ionizes only slightly in an aqueous solution. Such as a weak acid is termed a titration. Ka H3O A- HA where A- is the conjugate base of the acid.
CH3COOH is a carboxylic acid whose pKa is about 5 actually this one is 474. A weak acid HOCl is in solution with dissolved sodium hypochlorite NaOCl. B HI and HBr.
Which of the following is an example of weak acid. Which of the following is a buffer system. Considered to be a weak acid but still dissociates its proton significantly.
A strong acid is an acid that is completely ionized in an aqueous solution.

Solved Question 3 Which One Of The Following Is A Weak Acid Chegg Com

Solved Which Of The Following Is A Weak Acid D Hio4 A Chegg Com

Solved Which One Of The Following Is A Weak Acid H 2so 4 Chegg Com
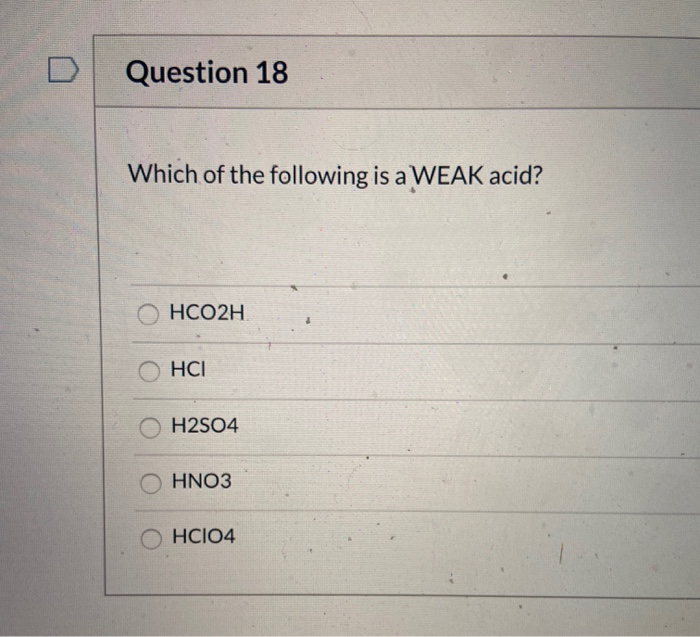
Solved Question 18 Which Of The Following Is A Weak Acid Chegg Com

Comments
Post a Comment